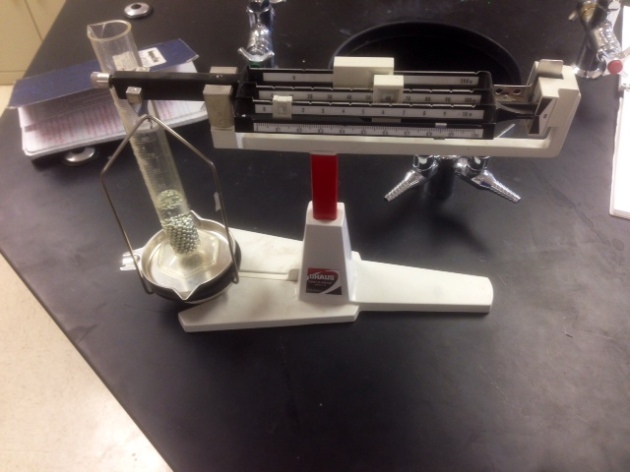
Today, we performed an exploration mini lab in which we attempted to find the composition of BB’s in a container through precision and accuracy. We began by taking a 100 mL graduated cylinder and filling it with 20 mL of water; then we found the mass of it on a balancing scale. We also found the mass of the graduated cylinder with 20 mL of water and with a volume of BB’s equal to 5 mL, 10 mL, and 15 mL. To get the final masses of the BB’s we subtracted the mass of the cylinder and water.
My groups results:
- 20mL+G.C.=60.6g
- 20mL of water+G.C.+5 mL BB’s=99.55g
- 20mL of water+G.C.+10 mL BB’s=139.55g
- 20mL of water+G.C.+15 mL BB’s=161.85g
The mass of each:
- 5 mL BB’s: 38.9g
- 10 mL BB’s: 78.95g
- 15 mL BB’s: 101.25g
Density of each:
- 5mL BB’s: 7.78 g/cm3
- 10 mL BB’s: 7.895 g/cm3
- 15 mL BB’s: 6.75 g/cm3
From this data, I can draw the conclusion that the BB’s are composed of Copper and Zinc. Copper has a density of 8.933 g/cm3 and zinc has a density of 7.134 g/cm3. These two averaged together to form a density of 8.0335 g/cm3, which is close to the average density from my experiment. My groups data is pretty reliable, but could have been more if we would have used the rules of significant digits. On our scale, we had the ability to measure to the 1/100th place, therefore, we had the ability to guess the 1/1000th place. We only took our data to the 1/100th place. This is a lack of precision. For example how a number such as 161.85 is not equal to and is less precise than 161.850, and that a greater number of zeros at the end of a number with a decimal means greater precision.


I like that you added each mass, density, and the results of each. One suggestion is to look up some more information on BB’s to see what other people have discovered about it! Very good blog!